Surgical science
The surgical science section is produced following a review of the best science journals published in English worldwide.

The NEON trial: nerve repair vs. alignment for digital nerve injuries
Justin C R Wormald, MRCS, DPhil
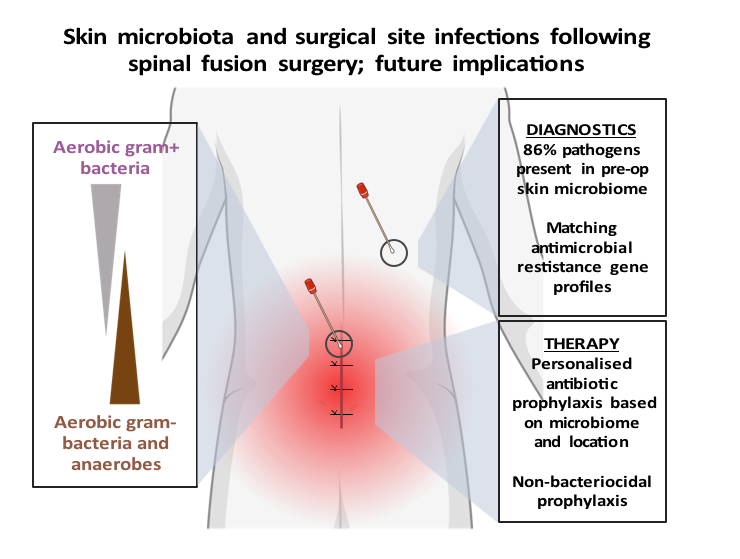
Review of: Contribution of the patient microbiome to surgical site infection and antibiotic prophylaxis failure in spine surgery
F.F. van den Berg1, M.A. Boermeester2
1Department of Medical Microbiology, Amsterdam UMC, Amsterdam, the Netherlands
2Department of Surgery, Amsterdam UMC, Amsterdam, the Netherlands
Article review Long, D. R. et al. Contribution of the patient microbiome to surgical site infection and antibiotic prophylaxis failure in spine surgery. Sci. Transl. Med.16, eadk8222 (2024). https://www.science.org/doi/10.1126/scitranslmed.adk8222

Exploring microbial engineering for enhanced mucosal healing in inflammatory bowel disease
Kamacay Cira, MD; Philipp-Alexander Neumann, MD
Department of Surgery, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany
Article Review

Are in vitro chip models the future of necrotizing enterocolitis research?
Ioannis A. Ziogas, MD, MPH, Ankush Gosain, MD, PhD Department of Pediatric Surgery, Children's Hospital of Colorado, Aurora, CO, 80045, USA
Article Review Lanik WE, Luke CJ, Nolan LS, et al. Microfluidic device facilitates in vitro modeling of human neonatal necrotizing enterocolitis-on-a-chip. JCI Insight. 2023;8(8):e146496. Necrotizing enterocolitis (NEC) is a severe, potentially fatal disease seen in premature neonates that results in intestinal injury and necrosis.1 The pathophysiology of NEC is based on loss of intestinal barrier integrity, translocation of bacteria across the gut barrier, and sepsis. The use of in vitro models is important to accelerate NEC research, given the shortage of surgically obtained samples from preterm neonates. The main limitation of currently available monotypic epithelial cell line in vitro models is the inaccurate simulation of the multiple cell types and complex intestinal dysbiosis implicated in NEC.2 On the other hand, the available intestinal organoid models can differentiate subtypes of intestinal epithelial cell and have apical-basolateral polarity within a three-dimensional spherical architecture. The limitation is the inability to assess the effect of microbial interactions or therapeutics on the apical epithelial surface.3 Notably, advances in microfluidic technology have led to the development of intestine-on-a-chip models that can simulate the human small intestine microenvironment through cellular differentiation, formation of three-dimensional villus-like axes, mucus production, continuous luminal flow, and mimicry of peristalsis.4

What happens to adipose tissue after obesity surgery.
David J. Leishman1 and Sayeed Ikramuddin1
1Department of Surgery, University of Minnesota, Minneapolis, MN, USA
Based on Dynamics of adipose tissue macrophage populations after gastric bypass surgery. Read the paper Download Author profiles

The hidden damage of brain injury after intracranial haemorrhage.
Jessie W Ho1, Zaiba Shafik Dawood1, Hasan B Alam1
1Department of Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Corresponding Author:
Hasan B. Alam, MD, FACS
Loyal and Edith Davis Professor of Surgery, and Professor of Cell & Developmental Biology
Chair, Department of Surgery, Feinberg School of Medicine, Northwestern University
Surgeon-in-Chief, Northwestern Memorial Hospital
Funding: None
Conflict of Interest: The authors declare no conflicts of interest
Data availability: N/A
This article highlights the close relation between the central nervous system and peripheral immune system, forming a neuroimmune axis that has clinical implications we are just beginning to understand. This article from the journal Science and Translational Medicine couples both patient data and a mouse model of intracranial haemorrhage (ICH) to explore the influence of ICH on haematopoietic stem cells (HSC) and the subsequent impact of HSCs on the brain. In the first experiment, bone marrow cells were harvested from skull flaps of patients requiring decompressive craniotomies for ICH and compared with cells from patients with unruptured aneurysms. In the second, ICH was induced in a mouse model by injection of autologous blood into brain parenchyma and subsequent harvest of femur bone marrow cells. Both the human and mouse data demonstrated increased myeloid progenitor cells and haematopoiesis with ICH. Adrenergic innervation through the β3 receptor on haematopoietic cells promotes production of Ly6Clow , a patrolling non-classical monocyte (NCM), in the bone marrow, which rapidly travels to the brain after ICH. Through transcriptomic analysis of the HSCs after ICH, Cdc42 (cell division cycle 42) was identified as an upregulated gene. Cdc42 was noted to be ablated in β3-adrenergic knockout mice, Adrb3-/-, linking the relationship of Cdc42 and adrenergic innervation. Treatment with a Cdc42 inhibitor led to decreased bone marrow proliferation, reduced Ly6Clow monocytes, and exacerbated brain injury. Given the role of the β3 receptor in proliferation and targeting of haematopoietic cells, mice were treated with a US Food and Drug Administration approved β3 receptor agonist, mirabegron. As expected, mirabegron treatment increased the Cdc42 activity in HSCs and increased Ly6Clow production and concentration in the brain. Most importantly mirabegron treatment reduced functional neurological deficits, perihaematomal oedema, and overall brain oedema after ICH. This article demonstrates that brain injury leads to a cascade of mechanisms modulated by β3-adrenergic innervation in which an NCM population provides protective effects to the brain. Lastly, the authors tested a potential targeted therapy upregulating the pathway and demonstrated improvement in brain injury outcomes.
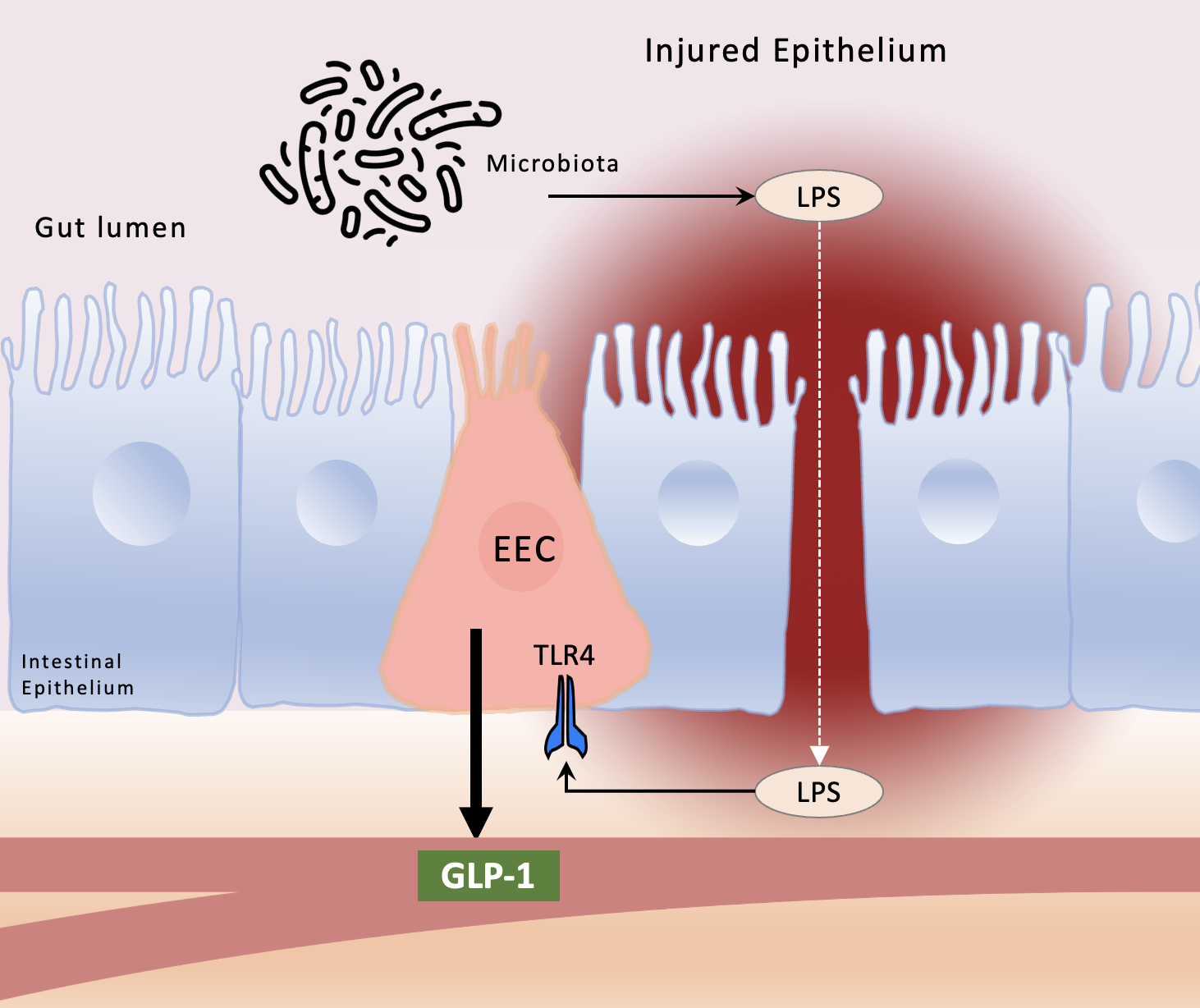
A surprising role for enteroendocrine cells and GLP-1 in regulating intestinal inflammation.
Carla Lopez, Chhinder P. Sodhi, David J. Hackam
Department of Surgery, Johns Hopkins University
Based on: Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros JP, Le Guern N, Plesnik J, et al. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017;21(5):1160-8. One of the most vexing issues in the field of inflammation research pertains to how the very bacteria and cells in the intestine that are required for host survival can also induce life-threatening inflammation. In its mildest form, inflammation in the gut may induce only brief discomfort or food intolerance, whereas in the extreme, gut inflammation can manifest as Crohn’s disease or ulcerative colitis, with life threatening implications. The precise factors that either accelerate or stop the development of inflammation in the intestine remain incompletely understood. The work of Lebrun et al now adds an important piece to this puzzle by describing a critical and previously unrecognized role for the gut-derived hormone glucagon-like-peptide 1 (GLP-1) in the regulation of intestinal inflammation. Enteroendocrine cells (EECs) are specialized epithelial cells that are responsible for the release of a variety of gut hormones. Chief amongst the various hormones that are secreted from the EECs is GLP-1, a peptide whose actions result in the regulation of circulating glucose, the control of appetite, and the motivation for food intake. Agonists of the GLP-1 receptor have been developed to treat type 2 diabetes, and recent studies suggest that such agonists may also have important effects in the control of obesity1, 2. In foundational studies leading up to the current work, other authors have focused on the fact that enteric bacteria produce lipopolysaccharide (LPS) that can influence gut hormone release3, 4. LPS mainly acts through the activation of toll-like receptor 4 (TLR4), a membrane bound receptor that serves as a convergence point for both infectious and non-infectious stimuli, triggering a proinflammatory response5. Under normal physiological conditions, several mechanisms restrict LPS signaling within the gut lumen; however, in inflammatory disease states, particularly when intestinal barrier integrity and function may be compromised, LPS can enter the circulation, where it can activate TLR4 on inflammatory cells and trigger a systemic inflammatory response.
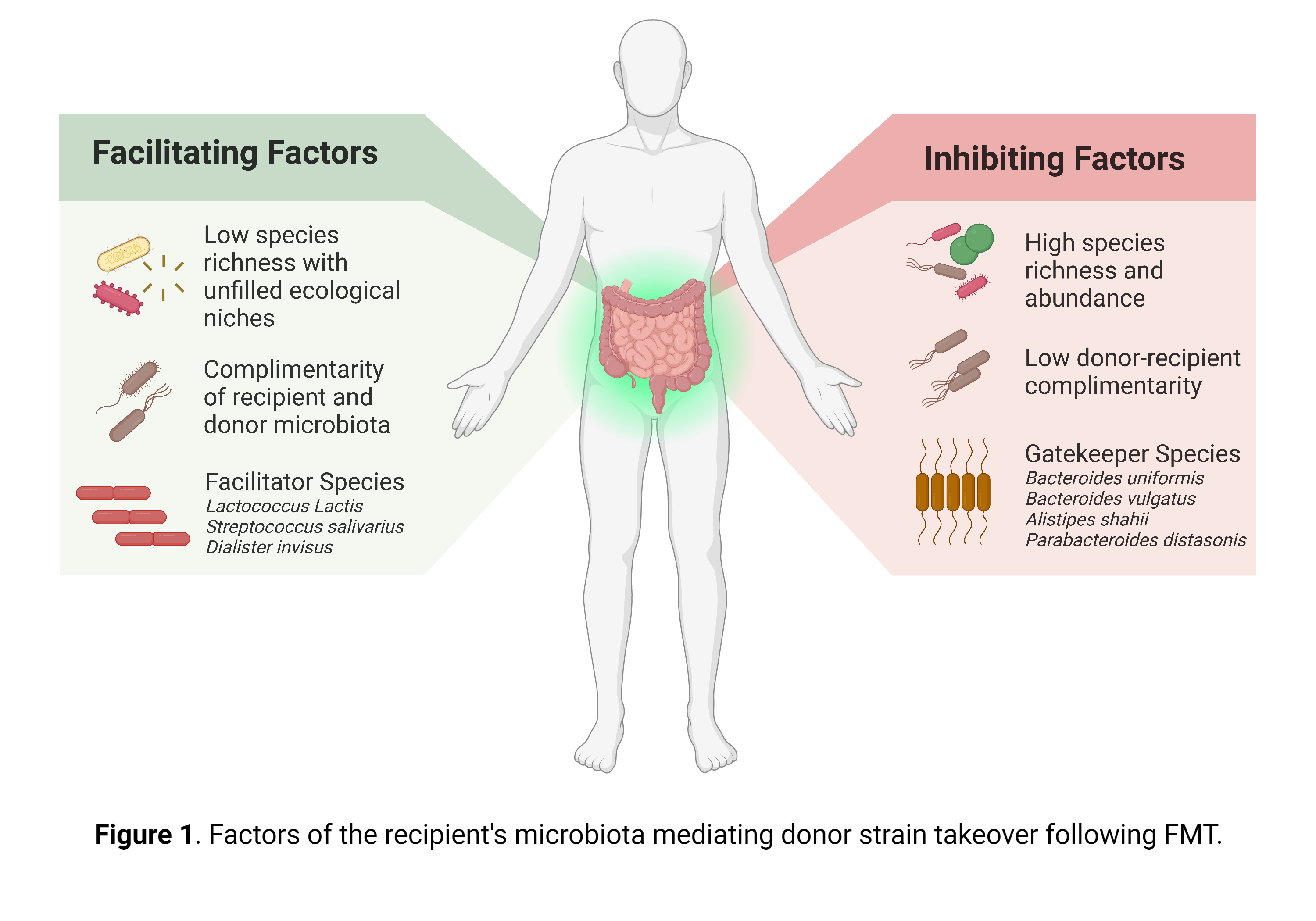
Faecal microbiota transplantation unravelled
Hugo Armand Roberto Sivov BA&Sc1, Florine Helene Zwezerijnen-Jiwa MD1,2,3, James Kinross MD, PhD1,*
1Department of Surgery and Cancer, St. Mary’s Hospital, Imperial College London, London W2 1NY, UK
2Tytgat Institute for Liver and Intestinal Research, Amsterdam Gastroenterology and Metabolism, Academic Medical Center, University of Amsterdam, 1105 BK Amsterdam, The Netherlands
3Department of Gastroenterology, Amsterdam Medical Centres, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands
*Corresponding Author:
Dr. James Kinross, department of Surgery and Cancer, St. Mary’s Hospital, Imperial College London, 10th Floor QEQMW, Praed Street, London, W2 1NY, UK
Funding Sources: Marie-Skłodowska-Curie grant agreement no. 814168.
Article Review Schmidt TSB, Li SS, Maistrenko OM, et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nature Medicine (2022). 28(9), 1902-1912 Faecal microbiota transplantation (FMT) is currently recommended in the UK by the National Institute for Health and Care Excellence (NICE) for the treatment of recurrent Clostridioidesdifficile infection (rCDI)1. Additionally, FMT is being extensively investigated for its potential application in surgical diseases, like the prevention of anastomotic leak2, as well as in ulcerative colitis (UC) and its related surgical complications, such as pouchitis3. Modulation of the faecal microbiome has significant implications for surgeons, as emerging evidence suggests pre-operative optimisation of the gut microbiota enhances patient outcomes and reduces surgical risks, such as surgical site infection, postoperative ileus and anastomotic leak4, 5, 6.

Discussion of: decellularized adipose matrices can alleviate radiation-induced skin fibrosis; adv wound care (New Rochelle).
Kanad Ghosh, MD1; Hannes Prescher, MD1; Summer E. Hanson, MD, PhD, FACS1
1Section of Plastic and Reconstructive Surgery, Department of Surgery, University of Chicago Medicine and Biological Sciences, Chicago, IL, 60637
With the advent of cellular targets and immunotherapy, cancer treatment has undergone significant improvement over the past several decades. While curative treatment of malignancy often relies on surgical excision, adjuvant modalities such as loco-regional irradiation remain important tools in comprehensive cancer care. Adjuvant radiotherapy (RT) is highly effective in reducing cancer burden, limiting the need for extensive surgery and decreasing the risk of local recurrence.1-3 However, RT brings collateral damage to the healthy surrounding soft tissues. Exposure to ionizing radiation results in a series of tissue changes marked by erythema, ulceration and oedema in the acute phase, followed by chronic inflammation and skin fibrosis, which may persist after treatment 4,5. As cancer survival rates continue to improve, an increasing number of patients are living with chronic morbidity related to RT. Autologous fat transfer (AFT) has emerged as a possible treatment to the harmful effects of irradiation.6,7 Here, adipose tissue is suctioned from one part of the body, processed and then injected in small aliquots directly into the irradiated tissues.8 The mechanism through which lipoaspirate exerts a reparative effect is poorly understood but thought to be through direct and indirect actions: direct differentiation of transferred adipose-derived stem cells (ASCs) into new adipocytes, and paracrine signalling of cytokines and growth factors (HGF, TGF-ß, FGF-1,2, VEGF) that inhibit profibrotic signalling pathways and contribute to the recruitment of proangiogenic cells 9. Like a skin graft, the adipose graft in AFT is dependent on the recipient tissue bed for nutrition and engraftment to achieve adequate ‘take.’ One of the challenges of fat grafting, in particular into a poorly perfused, irradiated tissue bed, is its unreliable retention rate, which is cited at between 30%-70%. Repeat procedures are often performed.10,11 As an alternative to AFT, decellularized adipose matrices (DAM) derived from discarded lipoaspirate have been developed. The allografts are processed through physical, chemical, and enzymatic purification techniques to develop decellularized scaffolds that retain the complex macromolecular architecture of the adipose tissue, and potentially its paracrine function via key growth factors retained in the graft. In recent studies, DAMs have been shown to promote adipose tissue regeneration, and have become a promising alternative to traditional fat grafting for soft tissue defects. 12 However, DAMs have not been studied in the context of radiation or a former tumour bed.
Creeping fat.
Alyson Kim BA1; Lillias H. Maguire, MD2,3
Affiliations:
1 Drexel University College of Medicine, Philadelphia, Pennsylvania
2 Department of Surgery, University of Pennsylvania, Philadelphia, Pennsylvania
3 Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
Creeping fat (CrF), migrating mesenteric adipose tissue (MAT), is a hallmark of Crohn’s Disease1 (CD) frequently associated with intestinal fibrosis. Surgeons operating for CD recognize CrF not only as a disease-specific phenomenon, but also as a technical challenge. Mesenteric transection is complicated in CrF as vessels retract with thickened, fibrotic fatty tissue resulting in haemorrhage and making haemostasis more difficult. Despite clinical familiarity with CrF however, its aetiology remains largely unknown. Additionally, it is unclear whether CrF represents an adaptive response in an attempt by the mesentery to seal off a “leaky” inflamed gut or is a pathological one, worsening the cycle of CD-related intestinal inflammation, scarring, stenosis, and ultimately development of surgical complications such as obstruction, perforation and fistula. This study sheds new light on CrF, explaining how this adipose tissue transitions from the traditional storage role to an active component, generating an adipogenic, fibrotic, and inflammatory environment. The authors hypothesize that CrF is a both a response to translocated gut microbiota and a driving force in fibrosis. They investigate these theories by identifying the bacterium Clostridium innocuum as a signature organism in CrF, characterizing CrF as a milieu distinguished by immune response and fibrosis, determining that C. innocuum gavage promotes this milieu and recapitulates CrF in gnotobiotic mice, and demonstrating in vitro that selective promotion of M2a macrophages may be the means by which C. innocuum drives fibrosis. The authors identify and focus on C. innocuum by first performing a metagenomic sequencing analysis on surgical samples of ileal tissue in CrF, grossly normal CD MAT, ulcerative colitis (UC) MAT, and healthy controls. The authors found that healthy MAT contains bacteria, suggesting that bacterial translocation to MAT is not in itself pathogenic. CD MAT, however, contained higher numbers of bacteria, but with lower biodiversity than healthy controls, consistent with mucosal data and previous studies2,3. 16S-RNA sequencing comparison to healthy MAT and UC-MAT identified a CD-specific expansion of the Erysipelotrichae lineage including C. innocuum. To assure the sequencing findings represented viable bacteria, the authors cultured MAT-derived bacterial isolates. Five bacteria were isolated as CD-specific and viable after cultivation.Among those, C. innocuum was most frequently isolated and selected for further study. A whole genome sequencing and comparative genomics study of C. innocuum revealed a conserved core of genes advantageous for translocation in adipose-like environments, such as protection against oxidative damage, cell motility, lipid detoxification, and evasion from immune functions and strain divergence between C. innocuum isolated from MAT and that isolated from mucosa. Factors potentially promoting viability in creeping fat also include type IV pili and mobility, lipid catabolism, and preference for b-hydroxybutyrate, a product of fatty acid oxidation.
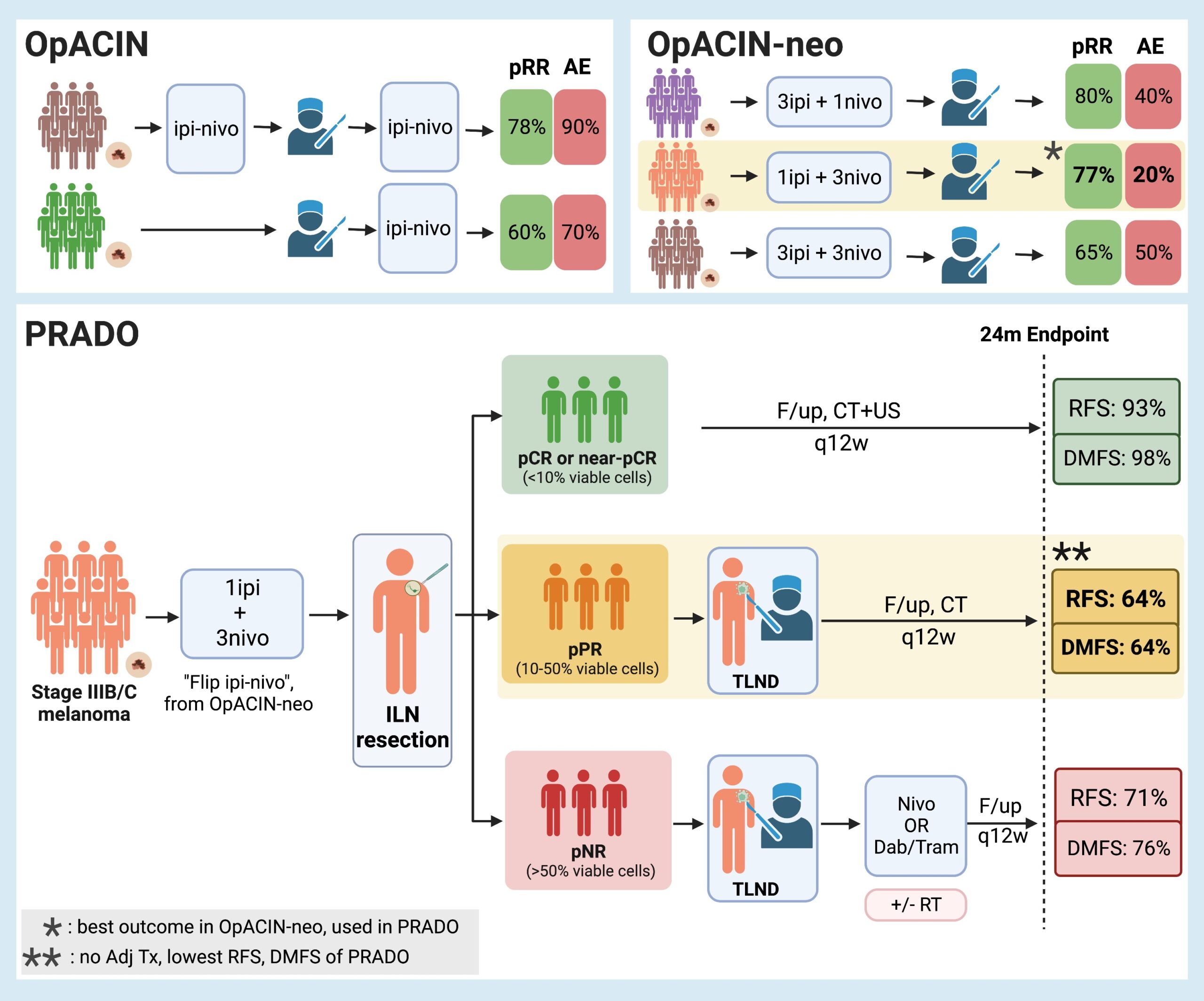
Personalized neoadjuvant immunotherapy for stage III malignant melanoma: notes on the PRADO study
1Aikaterini Dedeilia, M.D, 2Genevieve Boland, MD, PhD
1Postdoctoral Research Fellow, Surgical Oncology Research Laboratories; Department of Surgery, Massachusetts General Hospital; Research Fellow in Surgery, Harvard Medical School.
2Vice Chair of Research, Department of Surgery; Section Head, Melanoma/Sarcoma Surgery; Surgical Director, Termeer Center for Targeted Therapies; Director, Surgical Oncology Research Laboratories, Massachusetts General Hospital; Associate Professor of Surgery, Harvard Medical School.
Background While significant changes have occurred in the management of microscopic stage III disease due to the data from MSLT-21, the current standard of care for macroscopic stage III nodal malignant melanoma consists of initial surgical treatment with therapeutic lymph node dissection (TLND), followed by consideration for adjuvant therapy consisting of either anti-PD-1 monotherapy2 or BRAF/MEK inhibitors3. This results in improved relapse-free survival, but recurrence is still observed in almost half of the patients within 3-5 years4-6. Preclinical trials7-9 and emerging clinical data10 suggest that neoadjuvant immune checkpoint inhibition may have clinical benefit over adjuvant approaches. Given increasing enthusiasm for adjuvant and neoadjuvant approaches, the OpACIN (NCT02437279, phase I) and OpACIN-neo (NCT02977052, phase II) studies were established to investigate the safety and efficacy of neoadjuvant treatment with immune checkpoint inhibitors (ICI) combination, and to establish optimal dosing regimens to maximize clinical benefit while minimizing toxicity in patients with stage III melanoma. The OpACIN and OpACIN-neo trials

Can Clostridium Difficile infection be prevented?
Jason Xiao1, John Alverdy11Department of Surgery, University of Chicago Medicine, Chicago, IL, 60637
Paper for discussion: Fachi JL, Felipe JS, Pral LP, Silva BK, Correa RO, Cristiny M et al.Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019 Apr 16; 27: 750-761.e7. Although rare, Clostridium difficile-induced diarrhoea or colitis can complicate what otherwise appeared to be an uneventful elective operation. This rare, but potentially lethal complication results from multiple factors inherent to performing surgery, such as prolonged periods of starvation, antibiotic exposure, major physiological stress, and sleep deprivation1. C. difficile spores can spread easily, can resist multiple methods of decontamination and can remain viable for long periods of time. In many cases, the bacteria can remain hidden within the host’s gut microbiome and transferred to the healthcare setting by the patient themselves, rather than vice versa. While prevention is the best treatment, C. difficile infections (CDI) often prove resistant to antibiotics, and other modalities may be needed to restore homeostasis to the gut microbiome. Although faecal microbiota transplant has been proposed as a method for both prevention and treatment of CDI, even when severe colitis is present, many believe the most important action of the microbiome is to preserve its ability to produce key multifunctional metabolites 2. For example, the ability of the microbiota to produce the short-chain fatty acids (SCFAs) acetate, propionate, and butyrate has been identified to be an important therapeutic aspect in the prevention and treatment of CDI. SCFAs are absorbed by host intestinal epithelial cells (IECs) and participate in several immunoregulatory roles that influence the host response to inflammation and infection. Past studies have detected reduced SCFA concentrations, particularly butyrate, in patients with CDI3. Elevation of butyrate via dietary modulation or provision of SCFA-producing bacteria has been shown to attenuate CDI severity in animal studies4,5.

The microbiome and surgery: breakthrough or just hype?
John C. Alverdy MD FACS FSIS1, Benjamin Shogan MD2
1Sarah and Harold Lincoln Thompson Professor Executive Vice Chair
Chicago, Illinois, @JCAlvery,
2Department of Surgery, University of Chicago, Pritzker School of Medicine,
In 1980, the number of studies including the word “microbiome” was around eleven, today using microbiome as a search word in pubmed yields over 100,000 entries. For surgeons, the relevance of the gut microbiome lies in its promise to explain disease pathogenesis (i.e cancer, appendicitis, diverticulitis, surgical site infections) and treatment effects (antibiotic prophylaxis, bowel preparation, etc). Yet because the data output of a typical microbiome analysis can be vast, determining what is signal versus noise has become problematic. Similar to the early days when human gene chips became available, displays of massive datasets indicating that a patient in group A is “different” from patients in group B leaves readers skeptical. For example, when the human gene chip became available, the transcriptome (mRNA expression of nearly 20,000 protein coding genes) of human samples could be compared between patient samples. Yet these initial screens only described “differences” between groups of patients and failed to identify actionable items. The descriptive nature of these studies has forced some, for example, to completely question the genetic basis of cancer. Are we falling into the same trap with microbiome studies? Why microbiome studies are different. Claims that sequencing of the human genome was going to lead to major cures of complex diseases such as cancer have indeed been disappointing. First we were told that cancer is a genetic disease; once “junk” DNA turned out not to be junk, and once it became clear that gene-environment interactions (via histone modification?) played an important regulatory role in gene expression, the role of “lifestyle” became the new hype1. So where does that leave the microbiome in all of this? Issues such as how indoor and outdoor air quality, smoking, alcohol consumption, dietary choices, etc., influence one’s microbiome and then how in turn, its metabolites change host genetics is now under investigation. Perhaps one of the most striking examples of the power of microbiome analyses is a study examining the gut microbiome of 34 monozygotic twins discordant for multiple sclerosis; one twin suffered from the disease while the other did not2. Deep analysis of faecal samples from the discordant twins demonstrated clear differences; yet when samples were transferred into germ-free mice, only samples from the affected twin produced an encephalomyelitis-like picture whereas unaffected twin samples did not. The neurotoxic metabolites from the gut microbiome that play a role in this effect are now coming to light3. The fact that monozyotic twins are born with different fingerprints and the genomic identity in their microbiomes is highly variable should diminish our enthusiasm for interrogating host genes only4. Animals are holobionts, consisting of both host and microbial genes, each interacting with one another and with the environment. At the individual patient level, this presents major challenges to understand disease pathogenesis and its treatment.
